Malady: Consider doing a blood gas in hyponatremic patients, and think about pseudohyponatraemia if there’s a difference between formal electrolyte and VBG/ABG sodium. Adjust Na values for glucose. If they had a TURP recently, they’d tell you, it looks painful.
Take this with a grain of sodium…
Three commonly encountered ways of measuring sodium walk into the bar. First, the reference method – flame photometry, which relies on a neat bit of quantum mechanics where electrons dancing between levels of orbit emit a predictable spectrum, and concentration can be inferred from the intensity of light [1]. This was Genesis.
Decades later, ion-sensitive electrodes (ISE) emerged, of two different flavours. These gadgets work by measuring the potential difference between a reference electrode and those in the solution you have provided. First, we have blood gas analysers that use direct ISE which measure dissolved [Na+] in undiluted blood (i.e. reads [Na+] ion concentration within the water component of plasma). Large labs use indirect ISE where there is a dilution step and a reliance on the assumed composition of plasma – generally quoted as 93% water, 7% protein. The same issue of an assumed plasma composition and a dilution step also plagues flame photometry, since it has a narrow range of concentrations (to which plasma must be diluted) within which it is accurate [2].

This dilution step (that is not needed in direct ISE) and reliance on whole plasma volume, as opposed to the water fraction which is where the [Na+] concentration is physiologically relevant, is where the pseudo comes in. These analysers assume the 93:7 composition, and in cases where this ratio is upset, we run into systematic errors that are only magnified by the dilution factor. Common examples of the lost golden ratio include good going hyperlipidemia, and protein-rich states such as multiple myeloma, post-intravenous immunoglobulin (IVIg) and me after two scoops of whey protein. This also works the other way round – i.e. pseudo-hypernatremia – in protein-poor states, or may result in pseudo-normalisation of sodium in hypernatremic states.
The reason we use indirect ISE is one of cost-saving to prolong machine life, and reduce the amount required for sampling. The reason we use flame photometry is because it sounds cool dammit!
Here’s a worked example of this:
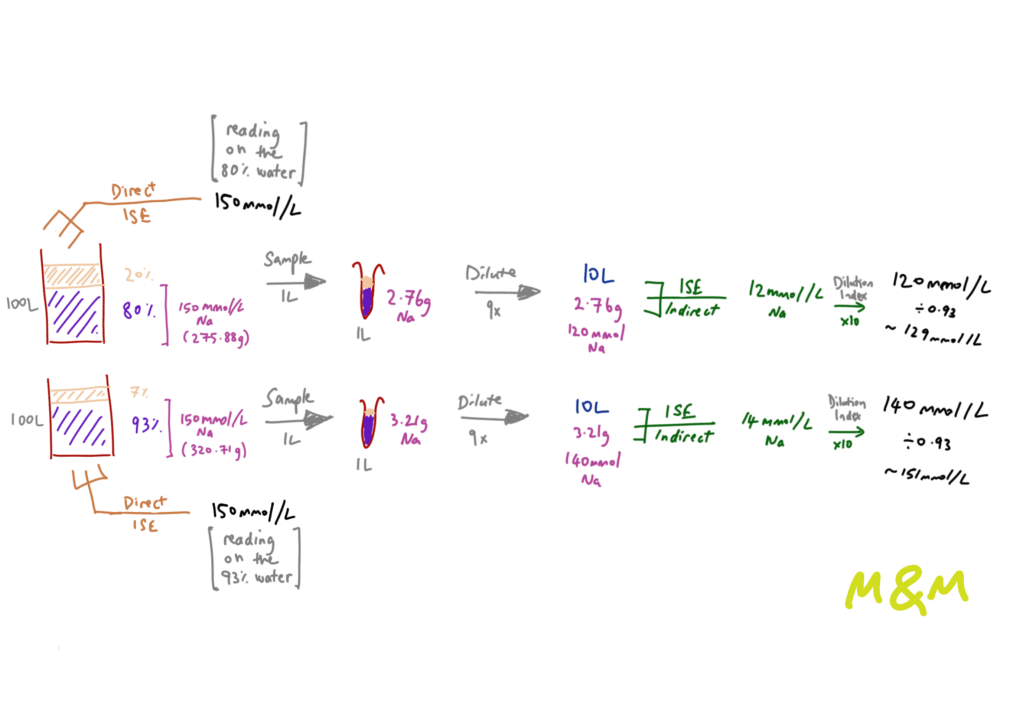
True hyponatremia is hypo-osmolar. If you suspect pseudohyponatremia, calculate the osmolality to see if it varies from measured osmolality (but note there are many other reasons for osmolar gaps) and generally normal osmolality is expected [3].
The issue of faulty measurements is not unique to sodium, but it is the most abundant and therefore most susceptible electrolyte to error. Similar principles apply to chloride too, but who knows what chloride actually does. The most clinically relevant perhaps is pseudohyperkalemia, where leucocytosis, thrombocytosis, vigorous shaking, processing time and looking at the sample the wrong way may lead to disastrous consequences.
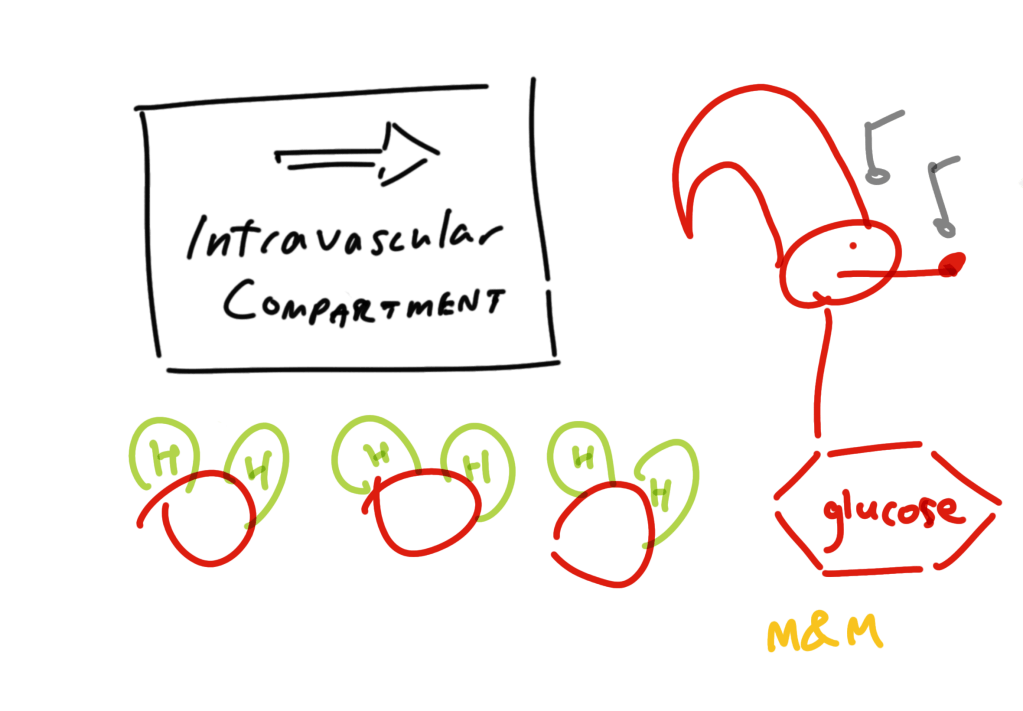
And an aside – there exist the hyperosmolar hyponatremias. Here the pathophysiology is one of needy osmolality where glucose draws water into the extracellular, intra-vascular compartment thereby diluting the present sodium. Other culprits (sometimes presenting as isotonic hyponatremia) are mannitol, and glycine which may be part of irrigation used in TURPs (trans-urethral resection of the prostate) and hysteroscopic procedures.
Now that you know everything that it is not, you can enjoy figuring out why they’re actually hyponatremic, which is conveniently not the scope of this article nor my practice.
Giants’ Shoulders:
[1] Overman and Davis’ ancient text in Journal of Biological Chemistry: https://www.sciencedirect.com/science/article/pii/S0021925817309237
[2] Aw and Kiechle, The American Journal of Emergency Medicine: https://www.sciencedirect.com/science/article/pii/0735675785900968
[3] Seguna et al’s very readable article in the Malta Medical School Gazette: Pseudohyponatraemia – A Literature Review | Malta Medical School Gazette
Others:
Electrolyte exclusion effect (whereby ions do not take occupy the solid parts of plasma): https://en.wikipedia.org/wiki/Electrolyte_exclusion_effect
American Journal of Kidney Diseases blog: Spurious Electrolytes in Malignancy
Katrangi et al’s real life analysis of this phenomenon: https://academic.oup.com/jalm/article/4/3/427/5636917
Dimeski G et al’s exhaustive chemistry masterclass about all the other errors I did not cover in Clinica Chimera Acta: https://www.sciencedirect.com/science/article/pii/S0009898109006287
Discover more from Myths & Maladies
Subscribe to get the latest posts sent to your email.
1 Comment
Comments are closed.